
LINX Device Recall and Lawsuit Information
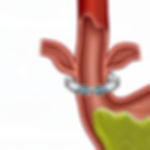
The U.S. Food and Drug Administration issued a recall of numerous Linx Reflux Management System devices after reports that a defect in the device was causing it to come apart. The Linx is a medical device implanted in patients diagnosed with Gastroesophageal Reflux Disease (GERD). It is comprised of a series of interlinked titanium beads held together by a wire band. The device is surgically placed around the patient's esophagus to prevent stomach acids from escaping.
The recall was issued after numerous reports that a defect in the device allowed the links to separate inside the patient’s body. Patients with defective devices are at risk of a recurrence of the GERD symptoms. The patient must have the device surgically removed. Patients who have had a LINX device and received a return of their symptoms and/or have been asked to have a revision surgery performed on it may be subject to the recall.
Frequently Asked Questions

Moving Forward With Your Case
Be sure to keep any medical-related bills, records, or directions from your healthcare providers as these may be important in proving your case. There are strict time deadlines for filing defective medical device lawsuits. If you miss the deadline, your claim could be barred forever.
Contact the Texas LINX reflux lawsuit lawyers at Sawicki Law Firm today! If your or someone you care about has been injured by a defective LINX Reflux system, contact our top-rated attorneys today to start on your case.
Our firm is currently handling claims regarding the Linx device. If you wish to find out more about your rights, and get your health back, please contact our firm at 888-468-8844 or answer our intake questionnaire here.
